Calllogenesis and phytochemical analysis of Euphorbia nutans Lag.
DOI:
https://doi.org/10.18387/polibotanica.60.20Keywords:
apices, chromatography, phytochemistry, leaves, metabolites.Abstract
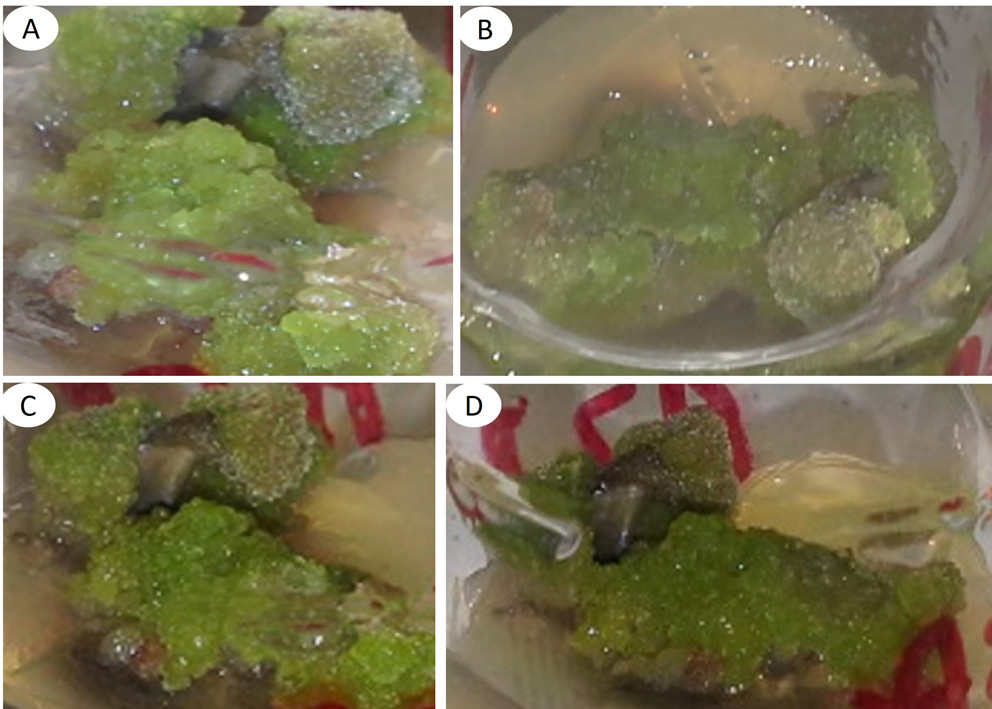
Euphorbia nutans is used to treat gastritis and external wounds empirically. It is intended to propagate in vitro but it is unknown if cellular dedifferentiation influences the biosynthesis of secondary metabolites. As an objective, it was proposed to obtain in vitro calli from E. nutans for phytochemical analysis, comparing the results with those obtained from wild plants. For this, Murashige and Skoog culture medium was used, adding cytokinins and auxins. The extracts were prepared under reflux with 3.41 g of calli, for two hours, using hexane, followed by dichloromethane and finally methanol. The phytochemical analysis of callus extracts was by comparison of secondary metabolites previously isolated from 3.534 Kg of dried and ground wild plant material of E. nutans, using thin layer chromatography (TLC) and 1H NMR spectroscopy. The in vitro variables were: percentage of response, weight, color and texture of callus. Callus weight was subjected to completely random analysis of variance and Tukey's test (p≤0.05). The results indicate that 100 % of leaf segments formed lemon green and friable calluses, with significantly higher weight (Tukey, p ≤0.05) using BA and NAA. TLC analysis revealed the presence of the same bands in callus extracts, as well as bands not found in wild plant extracts. The 1H NMR analysis allowed the identification of six secondary metabolites named as β-sitosterol, stigmasterol, α-amyrin acetate, β-lupeol, 24-methylene-cycloartan-3β-ol and an iridoid-type metabolite, being the first phytochemical study of E. nutans.
Keywords: apices, chromatography, phytochemistry, leaves, metabolites.
References
Aguilar Jiménez, D., Rodríguez De la O, J. L., Piña Guillén, J., & Silva Díaz, V. (2019). Propagación in vitro de Stevia rebaudiana y análisis preliminar de esteviósidos. Revista Mexicana De Ciencias Agrícolas, 10(1), 197-204. doi:10.29312/remexca.v10i1.1543.
Aguilar-Jiménez, D., & Rodríguez-De-la-O, J. L. (2020). Efecto de nitrato de plata en la germinación in vitro de Euphorbia nutans Lag. Biotecnología Vegetal, 20(4), 338-350.
Aguilar-Jiménez, D., Rodríguez-De-la-O, J. L., Reyes-Trejo, B., & Martínez-Solís, J. (2015). Respuestas morfogénicas in vitro y caracterización fitoquímica de la Euphorbia nutans Lag. Texcoco, Estado de México: Tesis de maestría, Universidad Autónoma Chapingo. Obtenido de https://repositorio.chapingo.edu.mx/handle/123456789/1255.
Albarrán-Mondragón, F. J., Orozco-Villafuerte, J., Mulia-Rodriguez, J., Hernández-Jaimes, C., Cruz-Sosa, F., & Buendía-González, L. (2022). Total phenolic content in fruits and in in vitro cultures of Bromelia karatas L. Revista Mexicana de Ingeniería Química, 21(1), Bio2685. doi:10.24275/rmiq/Bio2685.
Ali, S., & Tariq, A. (2013). Analysis of secondary metabolites in callus cultures of Momordica charantia cv. Jaunpuri. Biologia (Pakistan), 59(1), 23-32.
Alvarez-Aragón, C., Arzate-Fernández, A. M., Martínez-Martínez, S. Y., & Martínez-Velasco, I. (2020). REGENERACIÓN DE PLANTAS DE Agave marmorata Roezl, VÍA EMBRIOGÉNESIS SOMÁTICA. Tropical and Subtropical Agroecosystems, 23, #36. Obtenido de https://www.researchgate.net/publication/349107101.
Andújar, I., González, M., García-Ramos, J. C., Hajari, E., Bogdanchikova, N., Pestryakov, A. & Escalona, M. (2023). Are silver nanoparticles the “silver bullet” to promote diterpene production in Stevia rebaudiana? Plant Cell Tissue and Organ Culture, 155(2), 447-453. doi:10.1007/s11240-023-02450-5.
Arzate-Fernández, A. M., Martínez Velasco, I., Alvarez-Aragón, C., Martínez Martínez, S. Y., & Norman-Mondragón, T. H. (2020). RESPUESTA MORFOGENÉTICA DE DOS ESPECIES DE AGAVE REGENERADAS IN VITRO. Tropical and Subtropical Agroecosystems, 23, #47. Obtenido de https://www.researchgate.net/publication/342535024.
Baldi, A., Bisaria, V. S., & Srivastava, A. K. (2007). Biotechnological Approaches for the Production of some Promising Plant-Based Chemotherapeutics. En O. K. Quax, Medicinal Plant Biotechnology. From Basic Research to Industrial Applications (págs. 117-156). Weinheim, Alemania: WILEY-VCH Verlag GmbH & Co. KGaA. doi:10.1002/9783527619771.ch7.
Bhattacharyya, J., & Barros, C. B. (1985). Triterpenoids of Cnidosculus urens. Phytochemistry, 25(1), 274-276. doi:10.1016/S0031-9422(00)94550-3.
Bourgaud, F., Gravot, A., Milesi, S., & Gontier, E. (2001). Production of plant secondary metabolites: a historical perspective. Plant science, 161(5), 839-851.
Chaturvedula, V. S., & Prakash, I. (2012). Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J., 1(9), 239-242.
El-Naggar, L. J., & Beal, J. L. (1980). Iridoids. A review. Journal of Natural Products, 43(6), 649-707. doi:10.1021/np50012a001.
Fallah Ziarani, M., Tohidfar, M., & Navvabi, M. (2022). Modeling and optimizing in vitro percentage and speed callus induction of carrot via Multilayer Perceptron-Single point discrete GA and radial basis function. BMC Biotechnol, 22, 34. doi:10.1186/s12896-022-00764-4.
Gätjens-Boniche, O., Acuña-Matamoros, C. L., Montero-Carmona, W., Díaz, C., & Torres, S. (2018). Propagación masiva y formación de callos protocórmicos de Vainilla a partir de ápices radicales. POLIBOTÁNICA(45), 157-180. doi:10.18387/polibotanica.45.12.
Harborne, H. B. (1984). Phytochemical Methods (A guide to modern techniques of plant analysis) (Second edition ed.). New York: Chapman and Hall.
Hernández-Amasifuen, A. D., Argüelles-Curaca, A., Cortez-Lázaro, A. A., & Díaz-Pillasca, H. B. (2021). Inducción in vitro de callos a partir de explantes foliares en rocoto (Capsicum pubescens Ruiz & Pav.). La Granja: Revista de Ciencias de la Vida, 34(2), 131-140. doi:10.17163/lgr.n34.2021.09.
Ipav, S. S., Igoli, J. O., Tor-Anyiin, T. A., & Anyam, J. V. (2022a). Isolation and characterisation of lupeol and lupeol acetate from propolis obtained from Benue state. Journal of Chemical Society of Nigeria, 47(1). Obtenido de https://doi.org/10.46602/jcsn.v47i1.708.
Ipav, S. S., Igoli, J. O., Tor-Anyiin, T. A., & Anyam, J. V. (2022b). Isolation and characterisation of alpha and beta amyrins from propolis obtained from Benue state. Journal of Chemical Society of Nigeria, 47(2). Obtenido de https://doi.org/10.46602/jcsn.v47i2.723.
Isah, T. (2019). De novo in vitro shoot morphogenesis from shoot tip induced callus cultures of Gymnema sylvestre (Retz.) R.Br. ex Sm. Biological Research, 52(1), 3. doi:10.1186/s40659-019-0211-1.
Janarthanam, B., Gopalakrishnan, M., & Sekar, T. (2010). Secondary metabolite production in callus cultures of Stevia rebaudiana Bertoni. Bangladesh Journal of Scientific and Industrial Research, 45(3), 243-248.
Jasrai, Y., Thaker, K., & D´Souza, M. (2003). In vitro Propagation of Euphorbia pulcherrima Willd. Through Somatic Embryogenesis. Plant Tissue Cult., 31-36.
Kikuchi, T., Kadota , S., & Tsubono, K. (1986). Studies on the Constituents of Orchidaceous Plants. IV. Proton and Carbon 13 Signal Assignments of Cycloeucalenol –Tipe Triterpenes from Nervilia purpurea Schlechter by Two-Dimensional Nuclear Magnetic Ressonance Spectroscopy. Chemical and Pharmaceutical Bulletin, 34(6), 2479-2486.
Kondamudi, R., Sri R, M. K., & Pullaiah, T. (2009). Euphorbiaceae - a critical review on plant tissue culture. Tropical and Subtropical Agroecosystems, 10(3), 313-335.
Kreis, W. (2007). In vitro culturing techniques of medicinal plants. En O. Q. Kayser, Medicinal plant biotechnology. From basic research to industrial applications (págs. 157-185). Weinheim, Alemania: WILEY‐VCH Verlag GmbH & Co. KGaA. doi:10.1002/9783527619771.ch8.
Lee T, T., & Starratt A, N. (1972). Growth substance requirements and major lipid constituents of tissue cultures of Euphorbia esula and E. cyparissias. Canadian Journal Botany, 50, 723-726.
López-Ramírez, Y., Cabañas-García, E., Areche, C., Trejo-Tapia, G., Pérez-Molphe-Balch, E., & Gómez-Aguirre, Y. A. (2021). Callus induction and phytochemical profiling of Yucca carnerosana (Trel.) McKelvey obtained from in vitro cultures. Revista Mexicana de Ingeniería Química, 20(2), 823-837. doi:10.24275/rmiq/Bio2209.
Lozzi, A., Abdelwahd, R., Alami-Halimi, D., Mentag, R., & Abousalim, A. (2018). Optimization of a mature cotyledons-based in vitro culture system for embryogenic-callus induction in carob (Ceratonia siliqua L.). Revista Chapingo Serie Ciencias Forestales y del Ambiente, 25(1), 71-84. doi:10.5154/r.rchscfa.2018.06.053.
Mathur, J., & Hülskamp, M. (2002). Microtubules and microfilaments in cell morphogenesis in higher plants. Curr Biol., 12(19), R669-76. doi:10.1016/s0960-9822(02)01164-8.
Millones-Yamunaqué, A. M., Delgado-Paredes, G. E., Vásquez-Díaz, C., & Rojas-Idrogo, C. (2021). Callus induction, clonal propagation and in vitro germplasm conservation of ‘hualtaco’ Loxopterygium huasango Spruce ex Engl. (Anacardiaceae). Scientia Agropecuaria, 12(4), 545-556. doi:10.17268/sci.agropecu.2021.059.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco. Physiol. Plant., 15(3), 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x.
Naz, S., Tabassum, F., Javad, S., Ilyas, S., Aslam, F., Munir, N., & Ali, A. (2011). Micropropagation and callogenesis of a recalcitrant species Ricinus communis. Pak. J. Bot, 43(5), 2419-2422.
Perera, D., & Trader, B. (2010). Poinsettia ‘Prestige™ Red’ (Euphorbia pulcherrima) In Vitro Propagation. HortScience horts, 45(7), 1126-1128. doi:doi: 10.21273/HORTSCI.45.7.1126.
Pérez-Alonso, N., & Jiménez, E. (2011). Producción de metabolitos secundarios de plantas mediante el cultivo in vitro. Biotecnología Vegetal, 11(4), 195-211. Obtenido de https://revista.ibp.co.cu/index.php/BV/article/view/255/837.
Pickens, K., Cheng, Z., & Trigiano, R. (2005). Axillary bud proliferation and organogenesis of Euphorbia pulchurrima winter rose. In Vitro Cellular and Developmental Biology - Plant, 41(6), 770-774.
Pierik, R. L. M. (1990). Cultivo in vitro de las plantas superiores. Madrid-España: Mundi-Prensa.
Preciado Paredes, P. M., Ayala Astorga, G. I., & Martínez Heredia, D. (2016). Enraizamiento a partir de callos de Jatropha cuneata (wiggins & rollins) in vitro. CIBA Revista Iberoamericana De Las Ciencias Biológicas Y Agropecuarias, 4(8), 58-72. Obtenido de https://ciba.org.mx/index.php/CIBA/article/view/34.
Rangel-Estrada, S. D., Canul-Ku, J., Osuna-Canizales, F. J., García-Pérez, F., Del-Rosario-Montes, P., Vences-Hernández, A. B., & Hernández-Meneses, E. (2015). Regeneración in vitro de híbridos de nochebuena vía organogénesis. Revista Mexicana de Ciencias Agrícolas, 6(7), 1571-1585.
Ripley, K., & Preece, J. (1986). Micropropagation of Euphorbia lathyris L. Plant Cell, Tissue and Organ Culture, 5(3), 213-218.
Rodríguez Beraud, M. M., Latsague Vidal, M. I., Chacón Fuentes, M. A., & Astorga Brevis, P. K. (2014). Inducción in vitro de callogénesis y organogénesis indirecta a partir de explantes de cotiledón, hipocótilo y hoja en Ugni molinae. BOSQUE, 35(1), 111-118. doi:10.4067/S0717-92002014000100011.
Sakunphueak, A., & Panichayupakaranant, P. (2010). Increased production of naphthoquinones in Impatiens balsamina root cultures by elicitation with methyl jasmonate. Bioresour Technol., 101(22), 8777-8783. doi:10.1016/j.biortech.2010.06.067.
Šamaj, J., Baluška, F., & Hirt, H. (2004). From signal to cell polarity: mitogen‐activated protein kinases as sensors and effectors of cytoskeleton dynamicity. Journal of Experimental Botany, 55(395), 189-198. doi:10.1093/jxb/erh012.
Taiz, L., & Zeiger, E. (2006). Fisiología Vegetal (Tercera edición ed., Vol. II). Publicacions de la Universitat I de Catellón.
Vázquez-Baxcajay, L., Robledo-Paz, A., Muratalla-Lúa, A., & Conde-Martínez, V. (2014). Micropropagation of Stevia rebaudiana Bertoni, and steviosides detection. Bioagro, 26(1), 49-56.
Verpoorte , R., van der Heijden, R., & Memelink, J. (2000). Engineering the plant cell factory for secondary metabolite production. Transgenic Res., 9(4-5), 323-343. doi:10.1023/a:1008966404981.
Wu, Y., Ma, Y.-D., Li, Y., Zhang, L., & Xia, Y.-P. (2019). Plantlet regeneration from primary callus cultures of Lilium brownii F.E.Br. ex Miellez var. giganteum G. Y. Li & Z. H. Chen, a rare bulbous germplasm. In Vitro Cellular & Developmental Biology - Plant, 55(1). doi:10.1007/s11627-018-09955-1.
Xu, B., Dai, W., & Chao, W. (2008). An efficient method for in vitro regeneration of leafy spurge (Euphorbia esula L.). In Vitro Cell. Dev. Biol. - Plant, 44(6), 548-556. doi:doi: 10.1007/s11627-008-9139-9.
Downloads
Published
License
Copyright (c) 2025 POLIBOTANICA

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

Polibotánica by Departamento de Botánica de la Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional se distribuye bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.



















