Morphogenetic response of Agave angustifolia to the auxin-cytokinin gradient during the development of indirect somatic embryos
DOI:
https://doi.org/10.18387/polibotanica.60.18Keywords:
Agave angustifolia, callogenesis, somatic embryo, auxin, cytokininAbstract
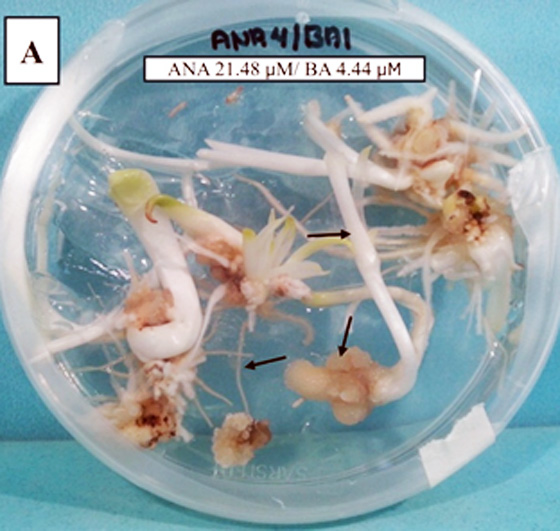
Agave angustifolia is a highly valuable species for the Mexican mezcal agroindustry. However, the increasing demand contrasts with the scarcity of high-quality germplasm and the low efficiency of conventional propagation methods. In this context, somatic embryogenesis emerges as a promising biotechnological strategy for the mass regeneration of elite plants under controlled conditions. The objective of this study was to evaluate the effect of three auxins (2,4-dichlorophenoxyacetic acid [2,4-D], indole-3-acetic acid [IAA], and naphthaleneacetic acid [NAA]) combined with benzylaminopurine (BA) on the induction of somatic embryogenesis in A. angustifolia, using zygotic embryo axes as explants. A completely randomized experimental design was applied to assess 27 hormonal combinations, derived from the factorial interaction of three concentrations of auxins (3.0, 4.0, and 5.0 mg L⁻¹) and three of BA (1.0, 2.0, and 3.0 mg L⁻¹). The induction medium for proembryogenic masses consisted of 25 % MS supplemented with 60 g L⁻¹ sucrose and L2 vitamins. Each zygotic embryo axis was considered an experimental unit. After 60 days of culture, the resulting calli were transferred to a histodifferentiation MS medium containing 0.1 mg L⁻¹ of the corresponding auxin and no BA. All cultures were kept in darkness for an additional 30–60 days to promote embryonic differentiation. Combinations of 2,4-D + BA induced callus formation in 71 % of the explants, with a predominance of embryogenic structures. The most effective combination was 5.0 mg L⁻¹ 2,4-D + 3.0 mg L⁻¹ BA, yielding between 1 and 36 somatic embryos per explant and an embryogenic efficiency of 17.6 ± 7.1. Combinations of NAA + BA mainly promoted rhizogenesis (87 %) with no embryo formation, while IAA + BA combinations were ineffective (≤ 10 %), resulting in oxidized tissues with no morphogenic development. Plantlets regenerated from somatic embryos germinated properly and exhibited a 100 % survival rate when acclimated under ex vitro conditions. The combination of 5.0 mg L⁻¹ 2,4-D and 3.0 mg L⁻¹ BA proved to be the most efficient for inducing somatic embryogenesis in Agave angustifolia. Among the tested auxins, 2,4-D showed a superior effect compared to NAA and IAA, establishing itself as the key regulator for inducing cellular totipotency. The high ex vitro acclimatization rate confirms the practical viability of the proposed protocol, with broad potential applications in conservation, genetic improvement, and sustainable agave production.
References
Abas, L., Kolb, M., Stadlmann, J., Janacek, D. P., Lukic, K., Schwechheimer, C., Sazanov, L. A., Mach, L., Friml, J., & Hammes, U. Z. (2021). Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proceedings of the National Academy of Sciences, 118(1), e2020857118. https://doi.org/10.1073/pnas.2020857118
Aguirre-Dugua, X., & Eguiarte, L. E. (2013). Genetic diversity, conservation and sustainable use of wild Agave cupreata and Agave potatorum extracted for mezcal production in Mexico. Journal of Arid Environments, 90, 36–44. https://doi.org/10.1016/j.jaridenv.2012.10.018
Alvarez-Aragón, C., Arzate-Fernandez, A.-M., Martínez-Martínez, S.-Y., & Martínez-Velasco, I. (2020). REGENERACIÓN DE PLANTAS DE Agave marmorata Roezl, VÍA EMBRIOGÉNESIS SOMÁTICA. Tropical and Subtropical Agroecosystems, 23(2). https://doi.org/10.56369/tsaes.3117
Álvarez-Ríos, G. D., Pacheco-Torres, F., Figueredo-Urbina, C. J., & Casas, A. (2020). Management, morphological and genetic diversity of domesticated agaves in Michoacán, México. Journal of Ethnobiology and Ethnomedicine, 16(1), 3. https://doi.org/10.1186/s13002-020-0353-9
Aronen, T., Varis, S., & Tikkinen, M. (2025). Somatic embryogenesis: concept, principles, and applications. En Forest Microbiology (pp. 373–388). Elsevier. https://doi.org/10.1016/B978-0-443-21903-0.00022-9
Ascough, G. D., & Fennell, C. W. (2004). The regulation of plant growth and development in liquid culture. South African Journal of Botany, 70(2), 181–190. https://doi.org/10.1016/S0254-6299(15)30234-9
Azizi, P., Rafii, M. Y., Maziah, M., Abdullah, S. N. A., Hanafi, M. M., Latif, M. A., Rashid, A. A., & Sahebi, M. (2015). Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mechanisms of Development, 135, 1–15. https://doi.org/10.1016/j.mod.2014.11.001
Bai, S., Chen, L., Yund, M. A., & Sung, Z. R. (2000). Mechanisms of plant embryo development. Current Topics in Developmental Biology, 50, 61–88. https://doi.org/10.1016/S0070-2153(00)50004-0
Batista-Silva, W., de Paiva Gonçalves, J., Siqueira, J. A., Martins, A. O., Ribeiro, D. M., Nunes-Nesi, A., Zsögön, A., & Araújo, W. L. (2024). Auxin metabolism and the modulation of plant growth. Environmental and Experimental Botany, 226, 105917. https://doi.org/10.1016/J.ENVEXPBOT.2024.105917
Bouchez, D., Uyttewaal, M., & Pastuglia, M. (2024). Spatiotemporal regulation of plant cell division. Current Opinion in Plant Biology, 79, 102530. https://doi.org/10.1016/j.pbi.2024.102530
Cárdenas-Aquino, M. D. R., Camas-Reyes, A., Valencia-Lozano, E., López-Sánchez, L., Martínez-Antonio, A., & Cabrera-Ponce, J. L. (2023). The Cytokinins BAP and 2-iP Modulate Different Molecular Mechanisms on Shoot Proliferation and Root Development in Lemongrass (Cymbopogon citratus). Plants, 12(20), 3637. https://doi.org/10.3390/plants12203637
Dávila, P., Arizmendi, M. D. C., Valiente‐Banuet, A., Villaseñor, J. L., Casas, A., & Lira, R. (2002). Biological diversity in the Tehuacán-Cuicatlán Valley, Mexico. Biodiversity and Conservation, 11(3), 421–442. https://doi.org/10.1023/A:1014888822920
Desai, P., Desai, S., Rafaliya, R., & Patil, G. (2022). Plant tissue culture: Somatic embryogenesis and organogenesis. En Advances in Plant Tissue Culture (pp. 109–130). Elsevier. https://doi.org/10.1016/B978-0-323-90795-8.00006-0
Domínguez-Rosales, M. S., Alpuche-Solís, Á. G., Vasco-Méndez, N. L., & Pérez-Molphe-Balch, E. (2008). EFECTO DE CITOCININAS EN LA PROPAGACIÓN in vitro DE AGAVES MEXICANOS. Revista Fitotecnia Mexicana, 31(4), 317. https://doi.org/10.35196/rfm.2008.4.317
Fehér, A. (2015). Somatic embryogenesis — Stress-induced remodeling of plant cell fate. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1849(4), 385–402. https://doi.org/10.1016/j.bbagrm.2014.07.005
Flores-Maya, S., Vargas-Jurado, M. Á., Suárez-Mota, M. E., & Barrera-Escorcia, H. (2015). Análisis cariotípico de Agave marmorata y A. peacockii (Agavaceae) ubicados en las terrazas aluviales del río Zapotitlán, Puebla, México. Polibotánica, 0(40). https://doi.org/10.18387/polibotanica.40.7
García-Mendoza, A. (2002). Distribution of agave (Agavaceae) in México.
Gulzar, B., Mujib, A., Malik, M. Q., Sayeed, R., Mamgain, J., & Ejaz, B. (2020). Genes, proteins and other networks regulating somatic embryogenesis in plants. Journal of Genetic Engineering and Biotechnology, 18(1), 31. https://doi.org/10.1186/s43141-020-00047-5
Gutiérrez-Mora, A., Rodríguez-Garay, B., Contreras-Ramos, S. M., Kirchmayr, M. R., & González-Ávila, M. (2014). SUSTAINABLE AND INTEGRAL EXPLOITATION OF AGAVE.
Jing, H., & Strader, L. C. (2019). Interplay of Auxin and Cytokinin in Lateral Root Development. International Journal of Molecular Sciences, 20(3), 486. https://doi.org/10.3390/ijms20030486
Li, D. Z., & Pritchard, H. W. (2009). The science and economics of ex situ plant conservation. Trends in Plant Science, 14(11), 614–621. https://doi.org/10.1016/J.TPLANTS.2009.09.005
Murashige, T., & Skoog, F. (1962). A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum, 15(3), 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Pérez‐Zavala, M. D. L., Hernández‐Arzaba, J. C., Bideshi, D. K., & Barboza‐Corona, J. E. (2020). Agave: a natural renewable resource with multiple applications. Journal of the Science of Food and Agriculture, 100(15), 5324–5333. https://doi.org/10.1002/jsfa.10586
Radoeva, T., Vaddepalli, P., Zhang, Z., & Weijers, D. (2019). Evolution, Initiation, and Diversity in Early Plant Embryogenesis. Developmental Cell, 50(5), 533–543. https://doi.org/10.1016/j.devcel.2019.07.011
Reyes-Díaz, J. I., Arzate-Fernández, A. M., & Piña-Escutia, J. L. (2018). Fuentes de sacarosa y nitrógeno orgánico influyen en la embriogénesis somática de Agave angustifolia. Revista Mexicana de Ciencias Agrícolas, 9(7), 1508–1513. https://doi.org/10.29312/remexca.v9i7.1676
Reyes-Díaz, J. I., Arzate-Fernández, A. M., Piña-Escutia, J. L., & Vázquez-García, L. M. (2017). Media culture factors affecting somatic embryogenesis in Agave angustifolia Haw. Industrial Crops and Products, 108, 81–85. https://doi.org/10.1016/j.indcrop.2017.06.021
Schaller, G. E., Bishopp, A., & Kieber, J. J. (2015). The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. The Plant Cell, 27(1), 44–63. https://doi.org/10.1105/tpc.114.133595
Su, Y. H., Liu, Y. B., & Zhang, X. S. (2011). Auxin-cytokinin interaction regulates meristem development. En Molecular Plant (Vol. 4, Número 4, pp. 616–625). Oxford University Press. https://doi.org/10.1093/mp/ssr007
Teale, W., & Palme, K. (2018). Naphthylphthalamic acid and the mechanism of polar auxin transport. Journal of Experimental Botany, 69(2), 303–312. https://doi.org/10.1093/jxb/erx323
Tian, R., Paul, P., Joshi, S., & Perry, S. E. (2020). Genetic activity during early plant embryogenesis. Biochemical Journal, 477(19), 3743–3767. https://doi.org/10.1042/BCJ20190161
Trejo, L., Soriano, D., Romano-Grande, E., Sánchez-Carmona, B., & Dávila-Navarro, D. E. (2024). Diversity of reproductive characters, seed set, and viability of Agave seeds used for pulque production and their wild relatives in Tlaxcala, Mexico. Genetic Resources and Crop Evolution, 71(6), 2877–2903. https://doi.org/10.1007/s10722-023-01803-5
Wang, F.-X., Shang, G.-D., & Wang, J.-W. (2022). Towards a hierarchical gene regulatory network underlying somatic embryogenesis. Trends in Plant Science, 27(12), 1209–1217. https://doi.org/10.1016/j.tplants.2022.06.002
Węgrzynowicz-Lesiak, E., Góraj, J., Miyamoto, K., Ueda, J., & Saniewski, M. (2013). Effects of auxin polar transport inhibitors on the growth of the excised fourth internode in tulips. Journal of Horticultural Research, 21(2), 31–39. https://doi.org/10.2478/johr-2013-0019
Zeng, F., Zhang, X., Cheng, L., Hu, L., Zhu, L., Cao, J., & Guo, X. (2007). A draft gene regulatory network for cellular totipotency reprogramming during plant somatic embryogenesis. Genomics, 90(5), 620–628. https://doi.org/10.1016/j.ygeno.2007.07.007
Zhang, W., Zhang, L., Jiang, W., Yang, H., Yang, T., Zhao, Y., Zhang, Z., & Ma, Y. (2025). DNA methylation regulates somatic stress memory and mediates plasticity during acclimation to repeated sulfide stress in Urechis unicinctus. Journal of Hazardous Materials, 487, 137264. https://doi.org/10.1016/J.JHAZMAT.2025.137264
Zizumbo-Villarreal, D., González-Zozaya, F., Olay-Barrientos, A., Platas-Ruíz, R., Cuevas-Sagardí, M., Almendros-López, L., & Colunga-GarcíaMarín, P. (2009). Archaeological Evidence of the Cultural Importance of Agave spp. in Pre-Hispanic Colima, Mexico. Economic Botany, 63(3), 288–302. https://doi.org/10.1007/s12231-009-9092-5
Downloads
Published
License
Copyright (c) 2026 POLIBOTANICA

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

Polibotánica by Departamento de Botánica de la Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional se distribuye bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.



















